Methane - a carbon atom bonded to 4 hydrogen atoms But carbon can bond to other carbon atoms in addition to hydrogen, as illustrated in the molecule ethane below: Ethane - a carbon-carbon bond. In fact, the uniqueness of carbon comes from the fact that it can bond to itself in many different ways. Carbon atoms can form long chains: Hexane - a 6. Carbon Atom stock pictures, royalty-free photos & images. Graphene 3D Graphene render. Hexagonal molecular structure. Scientific illustration or background. Carbon Atom stock pictures, royalty-free photos & images. Quantum-Dot Graphene Transistor A model of a quantum-dot transistor etched into a graphene sheet. Graphene is a sheet of one-atom.

Learning Outcomes
- Discuss why it is said that life is carbon-based and the bonding properties of carbon.
Carbon
Living things are carbon-based because carbon plays such a prominent role in the chemistry of living things. This means that carbon atoms, bonded to other carbon atoms or other elements, form the fundamental components of many, if not most, of the molecules found uniquely in living things. Other elements play important roles in biological molecules, but carbon certainly qualifies as the “foundation” element for molecules in living things. It is the bonding properties of carbon atoms that are responsible for its important role.
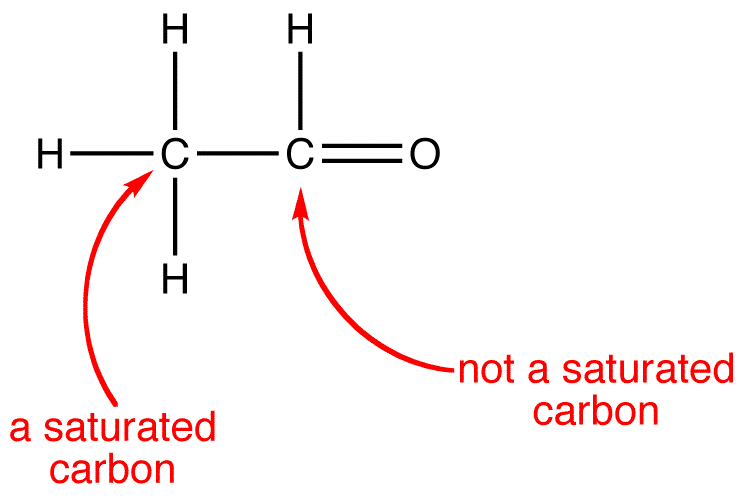

Carbon Bonding
Carbon Atom 13
The four covalent bonding positions of the carbon atom can give rise to a wide diversity of compounds with many functions, accounting for the importance of carbon in living things.
Carbon Atomic Mass
Carbon contains four electrons in its outer shell. Therefore, it can form four covalent bonds with other atoms or molecules. The simplest organic carbon molecule is methane (CH4), in which four hydrogen atoms bind to a carbon atom (Figure 1).
Carbon Atom
However, structures that are more complex are made using carbon. Any of the hydrogen atoms can be replaced with another carbon atom covalently bonded to the first carbon atom. In this way, long and branching chains of carbon compounds can be made (Figure 2a). The carbon atoms may bond with atoms of other elements, such as nitrogen, oxygen, and phosphorus (Figure 2b). The molecules may also form rings, which themselves can link with other rings (Figure 2c). This diversity of molecular forms accounts for the diversity of functions of the biological macromolecules and is based to a large degree on the ability of carbon to form multiple bonds with itself and other atoms.
Figure 2. These examples show three molecules (found in living organisms) that contain carbon atoms bonded in various ways to other carbon atoms and the atoms of other elements. (a) This molecule of stearic acid has a long chain of carbon atoms. (b) Glycine, a component of proteins, contains carbon, nitrogen, oxygen, and hydrogen atoms. (c) Glucose, a sugar, has a ring of carbon atoms and one oxygen atom.
Contribute!
